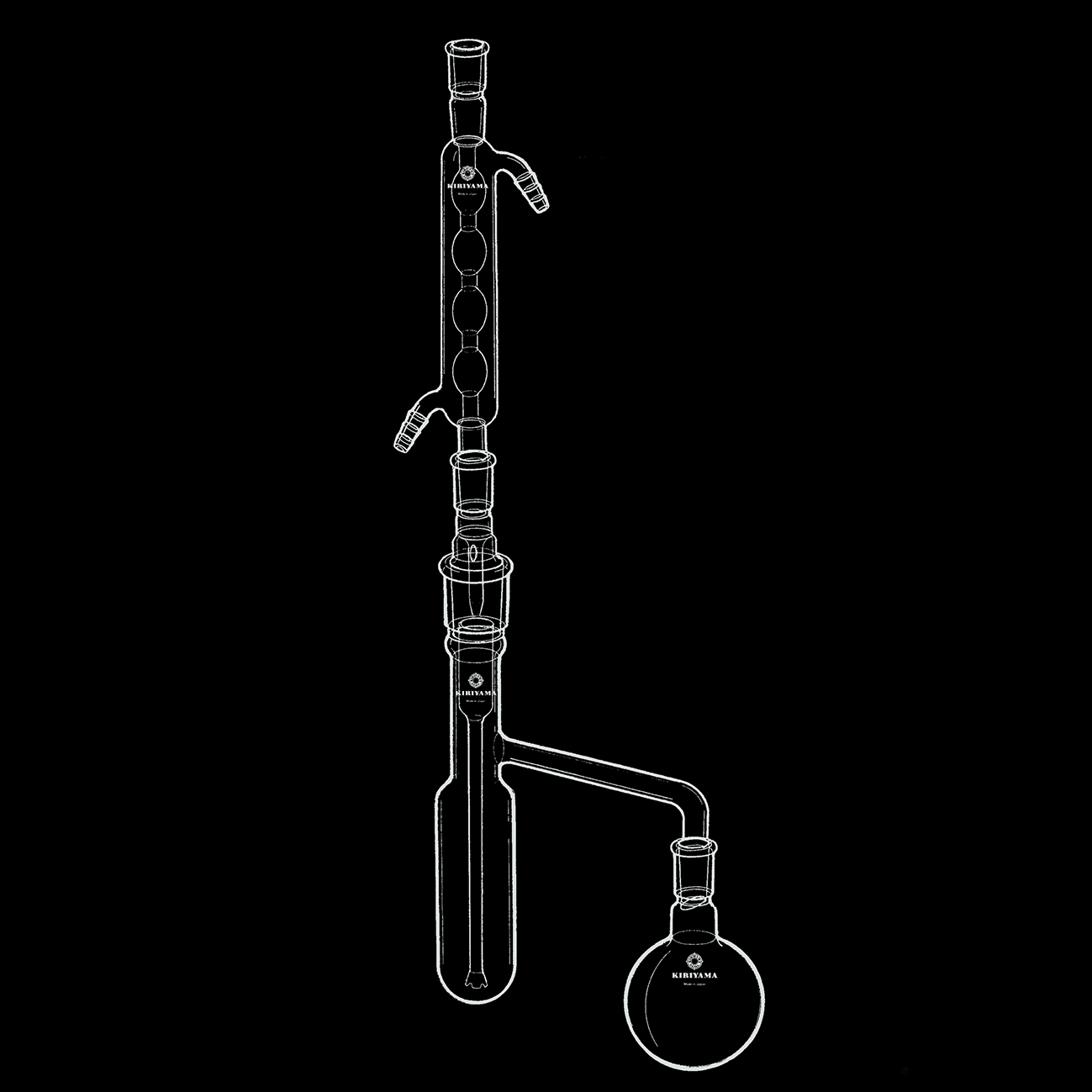
Liquid-liquid continuous extractor
This apparatus is for extracting organic substance dissolved in liquid, or for when sample changes from hydrophilic to hydrophobic by chemical reaction.
(CH3)3-C-OH + HCl ⇨ (CH3)3-C-Cl + H2O
t-butylalcohol t-butylchloride
〈 How to use when avobe reaction happens〉
- Put aqueous hydrochloric acid and t-butylalcohol mixture in the left vessel. Heat it up with stirring.
- Put hydrophobic solvent in the right flask and heat it up to vapour.
- Condensed solvent rise up through the bottom of the vessel. The solution with reacted butylchloride returns to flask.
- You could get condenced butylchloride after distilled off solvent.